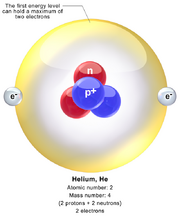
Helium Atom
Helium is a chemical element with symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table. Its boiling (4.222 K) and melting points (0.95 K) are the lowest among the elements and it exists only as a gas except in extreme conditions. Helium is one of the few elements with escape velocity, meaning that once released into the atmosphere, it escapes into space.
Helium is the second lightest element and is the second most abundant element in the universe, being present at about 24% of the total elemental mass, more than 12 times the mass of all the heavier elements combined. The vast majority of helium was formed by Big Bang nucleosynthesis one to three minutes after the Big Bang. Large amounts of new helium are being created by nuclear fusion of hydrogen in stars. It is produced in lesser amounts by the natural radioactive decay of heavy radioactive elements.
It is most often found in the thick atmosphere of hydrogen and helium surrounding a gas giant planet such as the planet Jupiter. On Earth it is relatively rare — 5.2 ppm by volume in the atmosphere.
HISTORY[]
Helium is named for the Greek god, Helios. It was first detected as an unknown yellow spectral line signature in sunlight during a solar eclipse in 1868 by French astronomer Jules Janssen. Janssen is jointly credited with detecting the element along with Norman Lockyer. Jannsen observed during the solar eclipse of 1868 while Lockyer observed from Britain. Lockyer was the first to propose that the line was due to a new element, which he named. The formal discovery of the element was made in 1895 by two Swedish chemists, Per Teodor Cleve and Nils Abraham Langlet, who found helium emanating from the uranium ore cleveite. In 1903, large reserves of helium were found in natural gas fields in parts of the United States.
USE[]
Historically, helium was used in cryogenics, particularly in the cooling of superconducting magnets, with the main commercial application being in medical scanners. Helium's other industrial uses—as a pressurizing and purge gas, as a protective atmosphere for arc welding and in processes such as growing crystals to make silicon wafers. A well-known but minor use is as a lifting gas in balloons and airships. Inhaling a small volume of helium temporarily changes the timbre and quality of the human voice. In scientific research, the behavior of the two fluid phases of helium-4 (helium I and helium II) was important to researchers studying quantum mechanics.
ISAH Engines of the third generation onwards function equally well on MOLGAS-He as it will on MOLGAS-W. It was the Antimatter process and the fuel handling systems that needed to be adapted, which they are from about 3100 onward when the so called 3rd generation of ISAH engines became available.
MOLGAS-He has never really caught on much, mainly due to the fact that the Fleet prefers MOLGAS-W, but it is still produced and mostly used by robotic ships and freighters as they buy whatever is cheapest and MOLGAS-He is usually available at Floating Refineries at a cheaper price.